Mar 21, 2023In order of increasing solubility in water, the following substances are ranked as CH₃ (CH₂)5OH < CH₃ (CH₂)3OH < CH₃CH₂CH2OH < CH₃CH₂OH (A<D<B<C). CH₃ (CH₂)5OH: It is n-hexanol, a six-carbon chain alcohol, is the least water-soluble. It has no polar functional groups, making it insoluble in water.
Arrange the following compounds in order of their expected increa… | Channels for Pearson+
The increasing order of solubility in water is C 6 H 5 N H 2 < (C 2 H 5) 2 N H < C 2 H 5 N H 2. The lower aliphatic amines are soluble in water because they are capable of forming hydrogen bonds with water. However, solubility decreases with increase in molar mass of amines due to increase in size of the hydrophobic alkyl part.
Source Image: numerade.com
Download Image
Find stepby-step Chemistry solutions and your answer to the following textbook question: Rank these substances in order of increasing solubility in water: A. $\mathrm CH_3 (CH_2)_5OH$ B. $\mathrm CH_3CH_2CH_2OH$ C. $\mathrm CH_3CH_2OH$ D. $\mathrm CH_3 (CH_2)_3OH$.
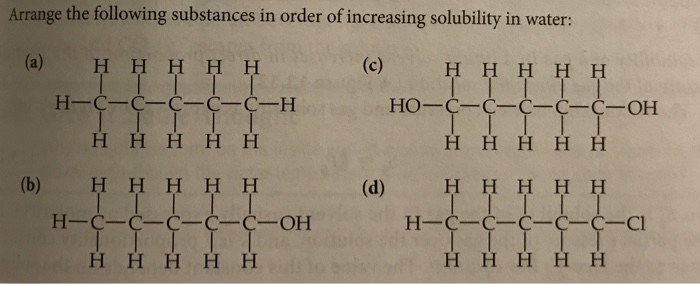
Source Image: chegg.com
Download Image
Solved List the following molecules in order of increasing | Chegg.com Mar 21, 2023Here are the substances provided: 1. CH₃CH₂OH (Ethanol) 2. CH₂CH₂CH₂OH (1-Propanol) 3. CH₃ (CH₂)₂OH (2-Propanol) 4. CH₂ (CH₂)₃OH (1-Butanol) Now let’s rank them in order of increasing solubility in water: Least soluble: 1-Butanol (CH₂ (CH₂)₃OH) – It has the longest hydrocarbon chain, making it the least soluble.
Source Image: opengeology.org
Download Image
Rank These Substances In Order Of Increasing Solubility In Water.
Mar 21, 2023Here are the substances provided: 1. CH₃CH₂OH (Ethanol) 2. CH₂CH₂CH₂OH (1-Propanol) 3. CH₃ (CH₂)₂OH (2-Propanol) 4. CH₂ (CH₂)₃OH (1-Butanol) Now let’s rank them in order of increasing solubility in water: Least soluble: 1-Butanol (CH₂ (CH₂)₃OH) – It has the longest hydrocarbon chain, making it the least soluble. Rank the following substances in order from most soluble in water to least soluble in water: octane, C8H18; propanol, C3H8OH; lithium nitrate, LiNO3; and pentane, C5H12. To rank items as equivalent, overlap them.
13 Crystal Structures – Mineralogy
Nov 10, 2023AI-generated answer To rank these substances in order of increasing solubility in water, we need to consider the structure and polarity of each substance. 1. CH3CH2CH2OH (propanol): This substance is a small alcohol molecule with a hydroxyl (-OH) group. Solved 3.48 Rank the following compounds in order of | Chegg.com
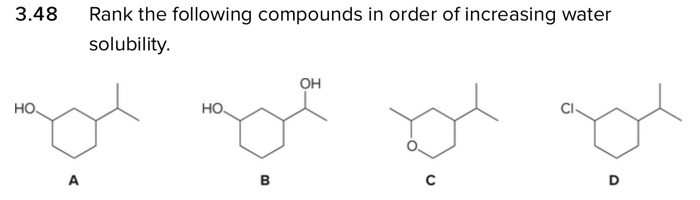
Source Image: chegg.com
Download Image
SOLVED: Rank these substances in order of increasing solubility in water: Substances (4 items) (Drag and drop into the appropriate area) Solubility Most soluble CH3CH2OH No more items CH2CH2CH2OH CH3(CH2)2OH CH3(CH2)3OH Least Nov 10, 2023AI-generated answer To rank these substances in order of increasing solubility in water, we need to consider the structure and polarity of each substance. 1. CH3CH2CH2OH (propanol): This substance is a small alcohol molecule with a hydroxyl (-OH) group.

Source Image: numerade.com
Download Image
Arrange the following compounds in order of their expected increa… | Channels for Pearson+ Mar 21, 2023In order of increasing solubility in water, the following substances are ranked as CH₃ (CH₂)5OH < CH₃ (CH₂)3OH < CH₃CH₂CH2OH < CH₃CH₂OH (A<D<B<C). CH₃ (CH₂)5OH: It is n-hexanol, a six-carbon chain alcohol, is the least water-soluble. It has no polar functional groups, making it insoluble in water.

Source Image: pearson.com
Download Image
Solved List the following molecules in order of increasing | Chegg.com Find stepby-step Chemistry solutions and your answer to the following textbook question: Rank these substances in order of increasing solubility in water: A. $\mathrm CH_3 (CH_2)_5OH$ B. $\mathrm CH_3CH_2CH_2OH$ C. $\mathrm CH_3CH_2OH$ D. $\mathrm CH_3 (CH_2)_3OH$.
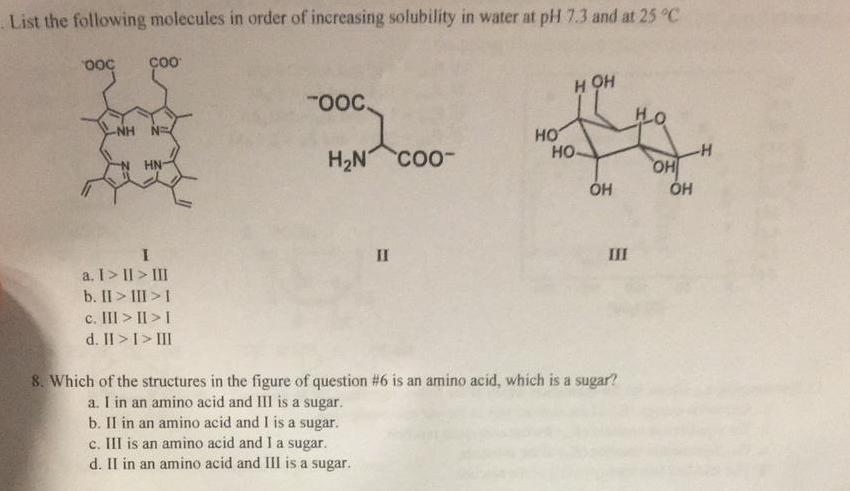
Source Image: chegg.com
Download Image
SOLVED: Arrange the following substances in order of increasing solubility in water. C5H12, C5H11Cl, C5H11OH, C5H10(OH)2 Chemistry questions and answers Rank these substances in order of increasing solubility in water. Solubility Substances (4 items) Drag and drop into the appropriate area) most soluble No more items CH-CH OH This problem has been solved! You’ll get a detailed solution from a subject matter expert that helps you learn core concepts. See Answer

Source Image: numerade.com
Download Image
Solubility Quick Assessment | PDF | Solubility | Chemistry Mar 21, 2023Here are the substances provided: 1. CH₃CH₂OH (Ethanol) 2. CH₂CH₂CH₂OH (1-Propanol) 3. CH₃ (CH₂)₂OH (2-Propanol) 4. CH₂ (CH₂)₃OH (1-Butanol) Now let’s rank them in order of increasing solubility in water: Least soluble: 1-Butanol (CH₂ (CH₂)₃OH) – It has the longest hydrocarbon chain, making it the least soluble.

Source Image: scribd.com
Download Image
The Solubility Of Organic Compounds In Water Rank the following substances in order from most soluble in water to least soluble in water: octane, C8H18; propanol, C3H8OH; lithium nitrate, LiNO3; and pentane, C5H12. To rank items as equivalent, overlap them.

Source Image: ozmo.io
Download Image
SOLVED: Rank these substances in order of increasing solubility in water: Substances (4 items) (Drag and drop into the appropriate area) Solubility Most soluble CH3CH2OH No more items CH2CH2CH2OH CH3(CH2)2OH CH3(CH2)3OH Least
The Solubility Of Organic Compounds In Water The increasing order of solubility in water is C 6 H 5 N H 2 < (C 2 H 5) 2 N H < C 2 H 5 N H 2. The lower aliphatic amines are soluble in water because they are capable of forming hydrogen bonds with water. However, solubility decreases with increase in molar mass of amines due to increase in size of the hydrophobic alkyl part.
Solved List the following molecules in order of increasing | Chegg.com Solubility Quick Assessment | PDF | Solubility | Chemistry Chemistry questions and answers Rank these substances in order of increasing solubility in water. Solubility Substances (4 items) Drag and drop into the appropriate area) most soluble No more items CH-CH OH This problem has been solved! You’ll get a detailed solution from a subject matter expert that helps you learn core concepts. See Answer