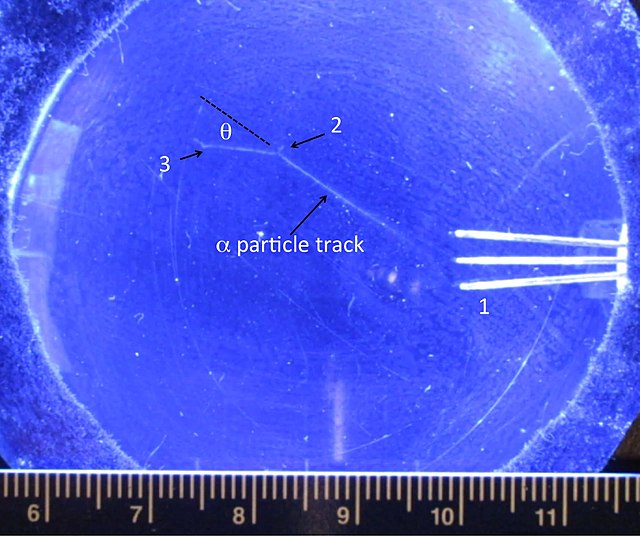
the number of alpha particles scattered out of a collimated beam upon hitting a thin metal foil. … and Marsden with the scattering of alpha particles by thin gold and silver foils (Phil. Mag. 25. 605 (1913), Figure 1). … although theα-particles may arrive in rapid succession. This pulse has a fast leading edge, and then a long trail
Alpha particles beamed at thin metal foil may A pass directly through without | Course Hero
A. alpha particles may pass directly through it without changing direction B. alpha particles may be slightly diverted by attraction to electrons C. alpha particles may be reflected by direct contact with nuclei D. A and C E. A, B, and C This problem has been solved!
Source Image: www.austintommy.com.ng
Download Image
A) Atoms of an element are not changed into different types of atoms by chemical reactions. B) All atoms of a given element are identical to each other and different from those other chemicals. C) During a chemical reaction, atoms are changed into atoms of different elements. D) Each element is composed of tiny, indivisible particles called atoms.
Source Image: en.wikipedia.org
Download Image
Meta Materials From UFOs | Metabunk In Rutherford’s apparatus, a narrow beam of alpha particles from a radon source (see diagram) fell upon a thin metal foil. A glass screen coated with zinc sulphide (which scintillates on absorbing alpha particles) was placed at the end of a travelling microscope and was used to detect scattered alpha particles.

Source Image: www.numerade.com
Download Image
Alpha Particles Beamed At Thin Metal Foil May
In Rutherford’s apparatus, a narrow beam of alpha particles from a radon source (see diagram) fell upon a thin metal foil. A glass screen coated with zinc sulphide (which scintillates on absorbing alpha particles) was placed at the end of a travelling microscope and was used to detect scattered alpha particles. directed at a thin piece of metal foil. Alpha particles are a type of nuclear radiation, traveling at about 1/10 the speed of light. As expected for such high-energy particles, most of the particles penetrated the thin metal foil and were detected on the other side. What was unexpected was that a few—a very few, to be sure—of the alpha
SOLVED: What will you observe when alpha particles are beamed at a thin metal foil? A. Alpha particles may pass directly through without changing direction. B. Alpha particles may be slightly diverted
Explanation: The correct answer is option d. A and C . When alpha particles are beamed at a thin metal foil, most of them will pass directly through without changing direction. However, some alpha particles may be reflected by direct contact with the nuclei of the metal atoms in the foil. Sleek Perspective Of Aluminum Foil Texture Background, Metal Pattern, Steel Plate, Iron Plate Background Image And Wallpaper for Free Download

Source Image: pngtree.com
Download Image
Alpha Particles in the Rutherford Scattering Experiment or Gold Foil Experiments Stock Vector – Illustration of radioactive, electrons: 184508754 Explanation: The correct answer is option d. A and C . When alpha particles are beamed at a thin metal foil, most of them will pass directly through without changing direction. However, some alpha particles may be reflected by direct contact with the nuclei of the metal atoms in the foil.

Source Image: www.dreamstime.com
Download Image
Alpha particles beamed at thin metal foil may A pass directly through without | Course Hero the number of alpha particles scattered out of a collimated beam upon hitting a thin metal foil. … and Marsden with the scattering of alpha particles by thin gold and silver foils (Phil. Mag. 25. 605 (1913), Figure 1). … although theα-particles may arrive in rapid succession. This pulse has a fast leading edge, and then a long trail

Source Image: www.coursehero.com
Download Image
Meta Materials From UFOs | Metabunk A) Atoms of an element are not changed into different types of atoms by chemical reactions. B) All atoms of a given element are identical to each other and different from those other chemicals. C) During a chemical reaction, atoms are changed into atoms of different elements. D) Each element is composed of tiny, indivisible particles called atoms.
Source Image: www.metabunk.org
Download Image
Solved Rutherford’s alpha particles beamed at thin metal | Chegg.com 4 min read. Rutherford’s gold foil experiment (Rutherford’s alpha particle scattering experiment) refers to an experiment carried out by Ernest Rutherford, Hans Geiger, and Ernest Marsden at the University of Manchester in the early 1900s. In the experiment, Rutherford and his two students studied how alpha particles fired at a thin piece

Source Image: www.chegg.com
Download Image
Mandala #182 – TrendyMandalas | OpenSea In Rutherford’s apparatus, a narrow beam of alpha particles from a radon source (see diagram) fell upon a thin metal foil. A glass screen coated with zinc sulphide (which scintillates on absorbing alpha particles) was placed at the end of a travelling microscope and was used to detect scattered alpha particles.
Source Image: opensea.io
Download Image
Rutherford’s alpha Scattering experiment showed that:the atoms were smashed up by alpha particlesthe atoms consisted of protons in a matrix of electronsthe nucleus was quite large and positively chargedthe nucleus was very directed at a thin piece of metal foil. Alpha particles are a type of nuclear radiation, traveling at about 1/10 the speed of light. As expected for such high-energy particles, most of the particles penetrated the thin metal foil and were detected on the other side. What was unexpected was that a few—a very few, to be sure—of the alpha

Source Image: www.toppr.com
Download Image
Alpha Particles in the Rutherford Scattering Experiment or Gold Foil Experiments Stock Vector – Illustration of radioactive, electrons: 184508754
Rutherford’s alpha Scattering experiment showed that:the atoms were smashed up by alpha particlesthe atoms consisted of protons in a matrix of electronsthe nucleus was quite large and positively chargedthe nucleus was very A. alpha particles may pass directly through it without changing direction B. alpha particles may be slightly diverted by attraction to electrons C. alpha particles may be reflected by direct contact with nuclei D. A and C E. A, B, and C This problem has been solved!
Meta Materials From UFOs | Metabunk Mandala #182 – TrendyMandalas | OpenSea 4 min read. Rutherford’s gold foil experiment (Rutherford’s alpha particle scattering experiment) refers to an experiment carried out by Ernest Rutherford, Hans Geiger, and Ernest Marsden at the University of Manchester in the early 1900s. In the experiment, Rutherford and his two students studied how alpha particles fired at a thin piece